
- HOME
- 產品介紹
- 半導體事業部
- Hamamatsu Photonics
- Lamp
- Hollow Cathode Lamps
- Hollow Cathode Lamps
Hollow Cathode Lamps
![]() +886-2-8772-8910
+886-2-8772-8910
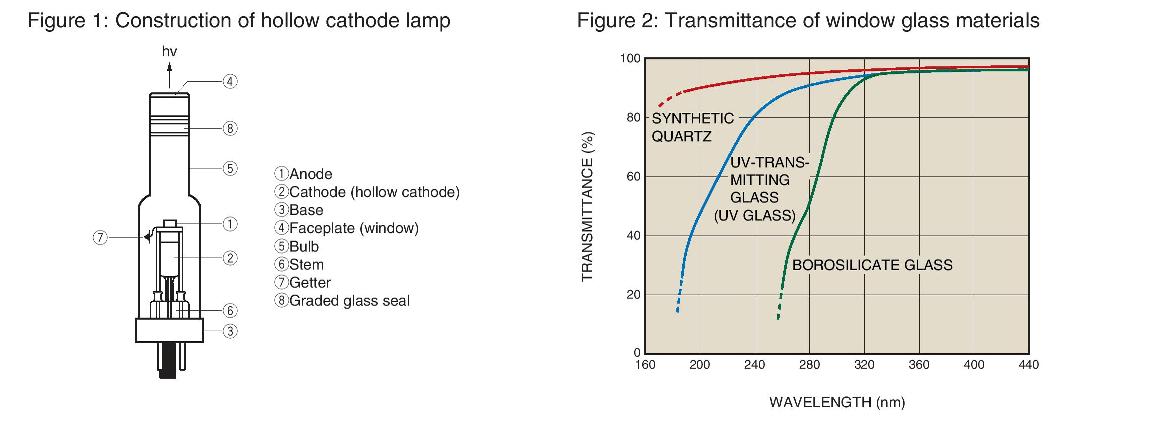
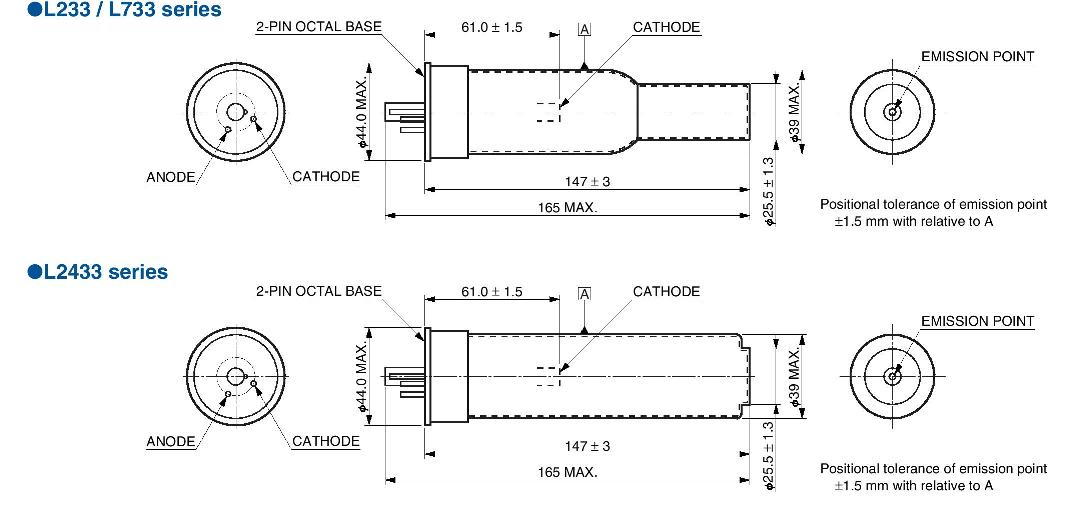
● L233 series (38 mm diameter): Single-element hollow cathode lamps (66 lamps) ①
|
Element |
Atomic |
Type No. |
Analytical Line |
Operating Current |
Maximum Current |
|
|---|---|---|---|---|---|---|
|
Ag |
Silver |
47 |
-47NB |
328.07 * |
10 |
20 |
|
Al |
Aluminium |
13 |
-13NB |
309.27 * |
10 |
20 |
|
As |
Arsenic |
33 |
-33NQ |
193.70 * |
10 |
12 |
|
Au |
Gold |
79 |
-79NQ |
242.80 * |
10 |
16 |
|
B |
Boron |
5 |
-5NQ |
249.68 * |
10 |
20 |
|
Ba |
Barium |
56 |
-56NB |
553.55 * |
10 |
20 |
|
Be |
Beryllium |
4 |
-4NQ |
234.86 * |
10 |
20 |
|
Bi |
Bismuth |
83 |
-83NQ |
223.06 * |
10 |
12 |
|
Ca |
Calcium |
20 |
-20NU |
422.67 * |
10 |
18 |
|
Cd |
Cadmium |
48 |
-48NQ |
228.80 * |
5 |
12 |
|
Co |
Cobalt |
27 |
-27NU |
240.73 * |
10 |
20 |
|
Cr |
Chromium |
24 |
-24NB |
357.87 * |
10 |
20 |
|
Cs |
Caesium |
55 |
-55NB |
852.11 * |
10 |
20 |
|
Cu |
Copper |
29 |
-29NB |
324.75 * |
10 |
20 |
|
Dy |
Dysprosium |
66 |
-66NB |
404.59 * |
15 |
15 |
|
Er |
Erbium |
68 |
-68NB |
400.79 * |
15 |
15 |
|
Eu |
Europium |
63 |
-63NB |
459.40 * |
15 |
15 |
|
Fe |
Iron |
26 |
-26NU |
248.33 * |
10 |
20 |
|
Ga |
Gallium |
31 |
-31NU |
287.42 |
4 |
6 |
|
Gd |
Gadolinium |
64 |
-64NB |
407.87 |
12 |
12 |
|
Ge |
Germanium |
32 |
-32NU |
265.16 * |
10 |
20 |
|
Hf |
Hafnium |
72 |
-72NU |
286.64 * |
20 |
25 |
|
Hg |
Mercury |
80 |
-80NU |
253.65 * |
4 |
6 |
|
Ho |
Holmium |
67 |
-67NB |
410.38 * |
15 |
20 |
|
In |
Indium |
49 |
-49NB |
303.94 * |
10 |
15 |
|
Ir |
Iridium |
77 |
-77NQ |
208.88 * |
20 |
20 |
|
K |
Potassium |
19 |
-19NB |
766.49 * |
10 |
15 |
|
La |
Lanthanum |
57 |
-57NB |
357.44 |
10 |
20 |
|
Li |
Lithium |
3 |
-3NB |
610.36 |
10 |
20 |
|
Lu |
Lutetium |
71 |
-71NB |
328.17 |
15 |
15 |
|
Mg |
Magnesium |
12 |
-12NU |
285.21 * |
10 |
18 |
|
Mn |
Manganese |
25 |
-25NU |
279.48 * |
10 |
20 |
|
Mo |
Molybdenum |
42 |
-42NB |
313.26 * |
10 |
20 |
|
Na |
Sodium |
11 |
-11NB |
589.00 * |
10 |
15 |
|
Nb |
Niobium |
41 |
-41NB |
334.91 * |
20 |
30 |
|
Nd |
Neodymium |
60 |
-60NB |
463.42 |
15 |
15 |
|
Ni |
Nickel |
28 |
-28NQ |
232.00 * |
10 |
20 |
|
Os |
Osmium |
76 |
-76NU |
290.90 * |
15 |
15 |
|
Pb |
Lead |
82 |
-82NQ |
217.00 * |
10 |
15 |
|
Pd |
Palladium |
46 |
-46NQ |
244.79 * |
10 |
20 |
|
Pr |
Praseodymium |
59 |
-59NB |
495.13 * |
15 |
15 |
|
Pt |
Platinum |
78 |
-78NU |
265.95 * |
10 |
20 |
|
Rb |
Rubidium |
37 |
-37NB |
780.02 * |
10 |
20 |
|
Re |
Rhenium |
75 |
-75NB |
346.05 * |
20 |
25 |
|
Rh |
Rhodium |
45 |
-45NB |
343.49 * |
10 |
20 |
|
Ru |
Ruthenium |
44 |
-44NB |
349.89 * |
20 |
25 |
|
Sb |
Antimony |
51 |
-51NQ |
217.58 * |
10 |
15 |
|
Sc |
Scandium |
21 |
-21NB |
390.74 |
10 |
15 |
|
Se |
Selenium |
34 |
-34NQ |
196.03 * |
20 |
25 |
|
Si |
Silicon |
14 |
-14NU |
251.61 * |
10 |
20 |
|
Sm |
Samarium |
62 |
-62NB |
429.67 * |
15 |
20 |
|
Sn |
Tin |
50 |
-50NQ |
224.61 * |
20 |
20 |
|
Sr |
Strontium |
38 |
-38NB |
460.73 * |
10 |
20 |
|
Ta |
Tantalum |
73 |
-73NU |
271.47 * |
10 |
20 |
|
Tb |
Terbium |
65 |
-65NB |
431.88 |
15 |
15 |
|
Te |
Tellurium |
52 |
-52NQ |
214.27 * |
10 |
15 |
|
Ti |
Titanium |
22 |
-22NB |
364.27 * |
10 |
20 |
|
Tl |
Thallium |
81 |
-81NU |
276.78 * |
7 |
10 |
|
Tm |
Thulium |
69 |
-69NB |
371.79 * |
10 |
15 |
|
V |
Vanadium |
23 |
-23NB |
306.64 |
10 |
20 |
|
W |
Tungsten |
74 |
-74NU |
255.14 * |
10 |
25 |
|
Y |
Yttrium |
39 |
-39NB |
410.23 * |
15 |
15 |
|
Yb |
Ytterbium |
70 |
-70NB |
346.43 |
10 |
10 |
|
Zn |
Zinc |
30 |
-30NQ |
213.86 * |
7 |
15 |
|
Zr |
Zirconium |
40 |
-40NB |
360.12 * |
20 |
20 |
|
D2 |
Hydrogen |
1 |
-1DQ |
240 |
30 |
35 |
● L733 series (38 mm diameter): Multi-element hollow cathode lamps (11 lamps)①
|
Element |
Atomic |
Type No. |
Analytical Line |
Operating Current |
Maximum Current |
|
|---|---|---|---|---|---|---|
|
Na-K |
Sodium |
11 |
-201NB |
Na 589.00 * |
10 |
15 |
|
Ca-Mg |
Calcium |
20 |
-202NU |
Ca 422.67 * |
10 |
18 |
|
Si-Al |
Silicon |
14 |
-203NU |
Si 251.61 * |
10 |
20 |
|
Fe-Ni |
Iron |
26 |
-204NQ |
Fe 248.33 * |
10 |
20 |
|
Sr-Ba |
Strontium |
38 |
-205NB |
Sr 460.73 * |
10 |
20 |
|
Al-Ca-Mg |
Aluminium |
13 |
-321NU |
Al 309.27 * |
10 |
18 |
|
Ca-Mg-Zn |
Calcium |
20 |
-322NQ |
Ca 422.67 * |
10 |
15 |
|
Cu-Mo-Co-Zn |
Copper |
29 |
-401NQ |
Cu 324.75 * |
10 |
15 |
|
Cd-Cu-Pb-Zn |
Cadmium |
48 |
-402NQ |
Cd 228.80 * |
10 |
15 |
|
Cu-Fe- Mn-Zn |
Copper |
29 |
-405NQ |
Cu 324.75 * |
8 |
15 |
|
Co-Cr-Cu- Fe-Mn-Ni |
Cobalt |
27 |
-601NQ |
Co 240.73 * |
10 |
20 |
● L2433 series (38 mm diameter): Single-element hollow cathode lamps (46 lamps)
|
Element |
Atomic |
Type No. |
Analytical |
Low ① |
High ① |
Accumulated ② |
Operating ② |
|
|---|---|---|---|---|---|---|---|---|
|
Ag |
Silver |
47 |
-47NB |
328.07 * |
10 |
400 |
20 000 |
500 |
|
Al |
Aluminium |
13 |
-13NB |
309.27 * |
10 |
600 |
30 000 |
500 |
|
As |
Arsenic |
33 |
-33NQ |
193.70 * |
12 |
500 |
7500 |
150 |
|
Au |
Gold |
79 |
-79NQ |
242.80 * |
10 |
400 |
20 000 |
500 |
|
B |
Boron |
5 |
-5NQ |
249.68 * |
10 |
500 |
5000 |
100 |
|
Ba |
Barium |
56 |
-56NB |
553.55 * |
15 |
600 |
30 000 |
500 |
|
Be |
Beryllium |
4 |
-4NQ |
234.86 * |
10 |
600 |
6000 |
100 |
|
Bi |
Bismuth |
83 |
-83NQ |
223.06 * |
10 |
300 |
6000 |
200 |
|
Ca |
Calcium |
20 |
-20NU |
422.67 * |
15 |
600 |
30 000 |
500 |
|
Cd |
Cadmium |
48 |
-48NQ |
228.80 * |
8 |
100 |
5000 |
500 |
|
Co |
Cobalt |
27 |
-27NU |
240.73 * |
15 |
400 |
20 000 |
500 |
|
Cr |
Chromium |
24 |
-24NB |
357.87 * |
10 |
600 |
12 000 |
200 |
|
Cu |
Copper |
29 |
-29NB |
324.75 * |
10 |
500 |
25 000 |
500 |
|
Dy |
Dysprosium |
66 |
-66NB |
404.59 * |
15 |
600 |
6000 |
100 |
|
Er |
Erbium |
68 |
-68NB |
400.79 * |
15 |
500 |
5000 |
100 |
|
Eu |
Europium |
63 |
-63NB |
459.40 * |
10 |
600 |
6000 |
100 |
|
Fe |
Iron |
26 |
-26NU② |
248.33 * |
12 |
400 |
20 000 |
500 |
|
Ga |
Gallium |
31 |
-31NU |
287.42 |
4 |
400 |
4000 |
100 |
|
Ge |
Germanium |
32 |
-32NU |
265.16 * |
20 |
500 |
5000 |
100 |
|
Hf |
Hafnium |
72 |
-72NU |
286.64 * |
20 |
600 |
6000 |
100 |
|
Hg |
Mercury |
80 |
-80NU |
253.65 * |
12 |
400 |
4000 |
100 |
|
Ho |
Holmium |
67 |
-67NB |
410.38 * |
10 |
600 |
6000 |
100 |
|
K |
Potassium |
19 |
-19NB |
766.49 * |
10 |
600 |
30 000 |
500 |
|
La |
Lanthanum |
57 |
-57NB |
357.44 |
20 |
600 |
9000 |
150 |
|
Li |
Lithium |
3 |
-3NB |
610.36 |
15 |
500 |
25 000 |
500 |
|
Mg |
Magnesium |
12 |
-12NU |
285.21 * |
10 |
500 |
25 000 |
500 |
|
Mn |
Manganese |
25 |
-25NU |
279.48 * |
10 |
600 |
30 000 |
500 |
|
Mo |
Molybdenum |
42 |
-42NB |
313.26 * |
10 |
600 |
9000 |
150 |
|
Na |
Sodium |
11 |
-11NB |
589.00 * |
10 |
600 |
12 000 |
200 |
|
Ni |
Nickel |
28 |
-28NQ |
232.00 * |
10 |
400 |
20 000 |
500 |
|
Pb |
Lead |
82 |
-82NQ |
217.00 * |
10 |
300 |
15 000 |
500 |
|
Pd |
Palladium |
46 |
-46NQ |
244.79 * |
10 |
300 |
3000 |
100 |
|
Pt |
Platinum |
78 |
-78NU |
265.95 * |
10 |
300 |
3000 |
100 |
|
Ru |
Ruthenium |
44 |
-44NB |
349.89 * |
20 |
600 |
6000 |
100 |
|
Sb |
Antimony |
51 |
-51NQ |
217.58 * |
15 |
500 |
7500 |
150 |
|
Se |
Selenium |
34 |
-34NQ |
196.03 * |
15 |
300 |
4500 |
150 |
|
Si |
Silicon |
14 |
-14NU |
251.61 * |
10 |
500 |
10 000 |
200 |
|
Sm |
Samarium |
62 |
-62NB |
429.67 * |
15 |
600 |
6000 |
100 |
|
Sn |
Tin |
50 |
-50NQ |
224.61 * |
20 |
500 |
25 000 |
500 |
|
Sr |
Strontium |
38 |
-38NB |
460.73 * |
10 |
500 |
25 000 |
500 |
|
Te |
Tellurium |
52 |
-52NQ |
214.27 * |
15 |
400 |
4000 |
100 |
|
Ti |
Titanium |
22 |
-22NB |
364.27 * |
10 |
600 |
12 000 |
200 |
|
V |
Vanadium |
23 |
-23NB |
306.64 |
10 |
700 |
7000 |
100 |
|
Y |
Yttrium |
39 |
-39NB |
410.23 * |
15 |
600 |
6000 |
100 |
|
Yb |
Ytterbium |
70 |
-70NB |
346.43 |
5 |
200 |
2000 |
100 |
|
Zn |
Zinc |
30 |
-30NQ |
213.86 * |
10 |
300 |
15 000 |
500 |
內置石墨爐原子吸收光譜儀;原子吸收光譜儀;Hollow Cathode Lamp;Atomic absorption spectroscopy;AAS;原子吸收光譜儀AAS;原子吸收光譜aa;原子放射光譜;火焰式原子吸收光譜儀;空心陰極燈管;中空陰極燈管;中空陰極管;空心陰極管;原子吸收分光光度法
